Prompt and personalized service meets customer expectations
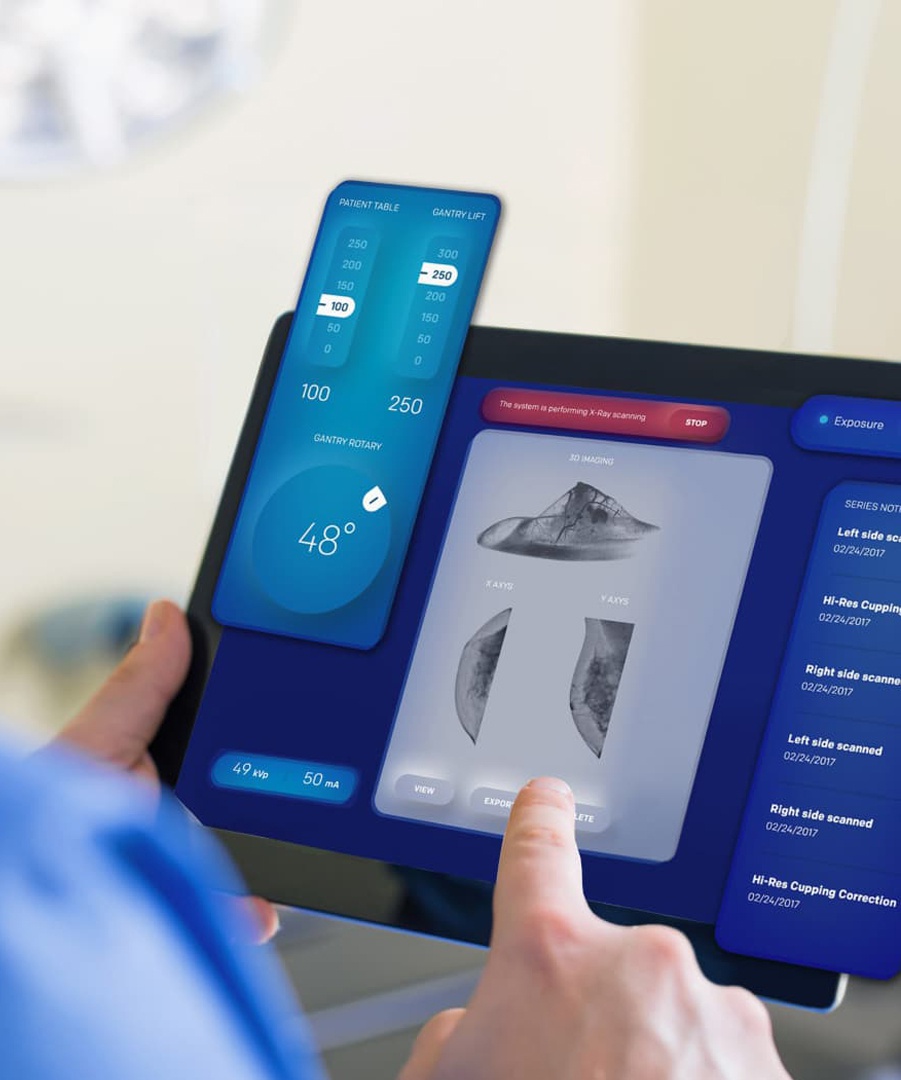

We are committed to delivering a high-quality and cost-effective range of products that enhance patient care and operational efficiency.
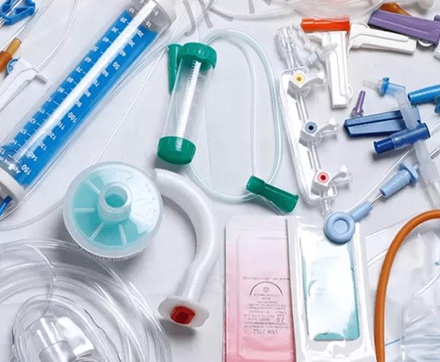

Offering OEM and ODM services for medical devices. This includes manufacturing products based on customer designs (OEM) and providing full-service design and production solutions (ODM) to meet customer-specific needs.
GOHEMED'S OEM & ODM SERVICES
Empowering Innovation and Growth in Medical Devices
In the medical device industry, innovation and efficiency are the keys to driving progress. With years of expertise in orthopedic implants, hemostasis and suturing devices, minimally invasive surgical instruments, and more, GOHEMED offers comprehensive OEM and ODM services to clients worldwide. We are committed to becoming your reliable partner, providing full-spectrum support from product design to manufacturing delivery for your medical device projects.
Flexible Solutions
Whether you have a mature design for an OEM project or need to start from scratch with an ODM collaboration, GOHEMED can adapt flexibly. We have a professional R&D team and advanced manufacturing facilities that can quickly adjust solutions according to your needs, ensuring smooth project progress. Our goal is to help you respond quickly in the highly competitive market and seize opportunities.
Efficiency and Quality Go Hand in Hand
At GOHEMED, we understand that the quality and safety of medical devices are of utmost importance. Therefore, we strictly adhere to international standards (such as ISO 13485) and industry regulations to ensure that every product undergoes rigorous quality control. At the same time, by optimizing our production processes and supply chain management, we ensure efficient delivery to help you shorten the product launch cycle and reduce operational costs.
Deep Collaboration and Continuous Support
By choosing to partner with GOHEMED, you will receive more than just manufacturing services—you will gain a long-term partner. We offer full-process services from design verification, manufacturing, to after-sales support, ensuring that your project is professionally guided and safeguarded at every stage. Our team will work closely with you to jointly address market changes and technological challenges, helping your brand grow continuously.
GOHEMED is committed to becoming your most trusted partner. Whether it is OEM or ODM services, we will provide you with professional and efficient support to contribute to the development of global healthcare.
Some products have attracted attention and are well-received in the market. We will continue to consolidate and improve them to reward the trust of customers.

Comprehensive Solution Provider for Medical Device Production
Contact Us

Prompt and personalized service meets customer expectations

Rigorous quality checks ensure top-notch product standards

Efficient cost management offers competitive pricing

Flexible OEM/ODM solutions meet diverse customer needs
Recently, Changying Medical was officially recognized as an “Innovative Small and Medium-sized Enterprise” by the competent authority of industry and information technology. This prestigious certification is a strong endorsement of the company’s capabilities in technological innovation, specialization, and growth potential. It also marks another solid step forward in Changying Medical’s journey of innovation within the medical device industry. The “Innovative SME” certification is a national initiative aimed at encouraging small and medium-sized enterprises to increase investment in research and development and enhance their core competitiveness. Since its establishment, Changying Medical has remained committed to technology-driven growth, focusing on the R&D and manufacturing of implantable medical devices in dentistry, orthopedics, and neurosurgery. The company has continuously introduced high-quality, market-competitive products that serve medical clients both domestically and internationally. This recognition reflects the company’s comprehensive strength in product innovation, management systems, and intellectual property. Looking ahead, Changying Medical will continue to increase its R&D investment, strengthen its independent innovation capabilities, and actively play a leading role in the industry—contributing to the high-quality development of the healthcare sector.
 May 16, 2025
May 16, 2025Dear Valued Customers, As Labor Day approaches, our team will be taking a short break from May 1st to May 5th, 2025. During this period, our factory operations will be paused. However, we will remain available for any urgent matters that may arise. Important Notes During the Holiday 1. Orders & Inquiries: Orders placed before the holiday will be processed promptly when we return. For urgent matters, please email us at info@gohemed.com. We will monitor our inbox and respond as quickly as possible. 2. Shipping: Due to the holiday, there may be slight delays in shipments. Rest assured, we will prioritize any pending deliveries as soon as normal operations resume. 3.Emergency Support: If you require urgent assistance during the holiday, please don't hesitate to reach out. While response times may be a little longer, we will ensure that no critical issues are left unaddressed. Post-Holiday Operations Normal operations will resume on May 6th, 2025, and we will prioritize addressing any backlog of inquiries. We appreciate your patience and understanding during this time. Thank you for your continued trust in our products and services. Wishing you a safe and relaxing Labor Day holiday!
 April 30, 2025
April 30, 2025Recently, Xiamen Changying Medical Technology Co., Ltd., an affiliate and partner of GOHEMED, announced that after long-term unremitting efforts and continuous investment in research and development, the company's main product line has achieved significant expansion, launching a series of Class III orthopedic medical device products. The new product line includes orthodontic mini-implants, rib plate systems, subtalar joint stabilization screws, craniofacial bone plates, and craniofacial bone screws. Each product has been developed in strict accordance with rigorous manufacturing processes and industry standards, and has been approved and certified by China's highest regulatory authority in the medical industry, the National Medical Products Administration (NMPA), ensuring the safety, reliability, and superior performance of the products. Ms. Chen, the company's manager, stated: "Our goal is to provide medical professionals with effective tools and supplies to help them offer higher standards of medical care to patients. These new products aim to improve the efficiency and effectiveness of medical procedures, while focusing on product safety and patient experience." The launch of this series of products also aligns with the company's broader strategy to expand its influence in the domestic and international medical device markets. GOHEMED will collaborate with Xiamen Changying Medical Technology Co., Ltd., planning to work with hospitals and medical institutions around the world to integrate these medical device products into their operations. These new products are expected to simplify related medical practices and support medical staff in providing excellent patient care, marking an important milestone in the company's continuous innovation and development journey.
 November 01, 2024
November 01, 2024GOHEMED will participate in the 90th CMEF 2024 from October 12-15 in Shenzhen. Welcome to visit our booth M39, Hall13.
 September 18, 2024
September 18, 2024